Liver imaging
Gadoxetate DCE-MRI biomarkers for liver transporter function, DDI and DILI assessment.
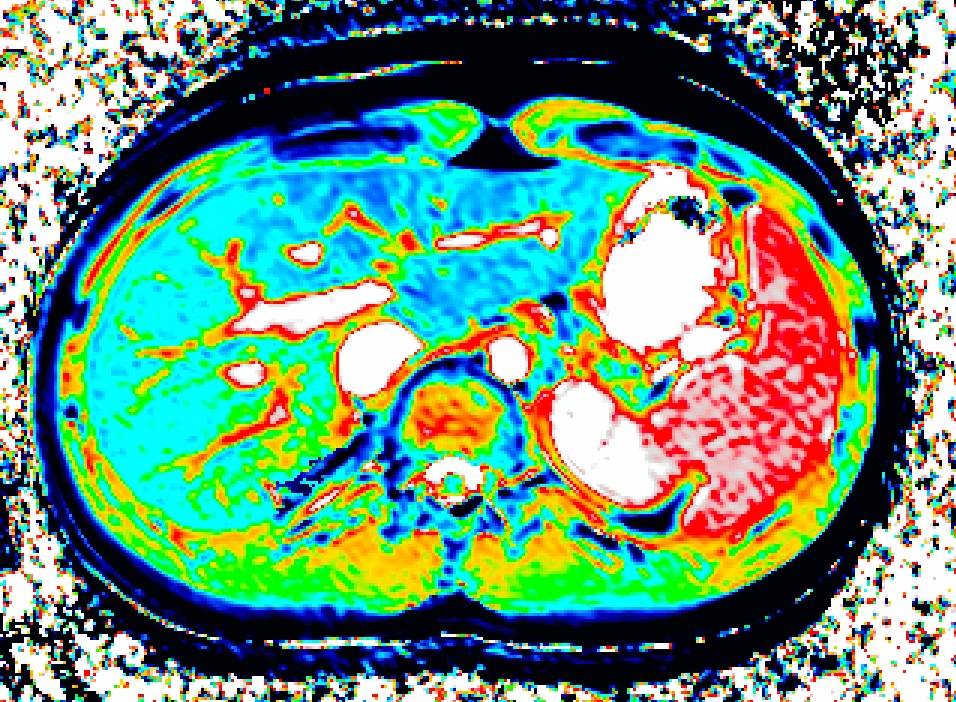
Bioxydyn offers MRI measurement of liver transporter fluxes using dynamic contrast-enhanced MRI (DCE-MRI), plus other imaging biomarkers, including relaxation time measurements, in the investigation of liver diseases.
Bioxydyn is the lead commercial partner in the IMI TRISTAN project, which is investigating the liver and lung toxicity of many drugs in use today with the goal of validating the use of imaging biomarkers to assess and predict toxicity.
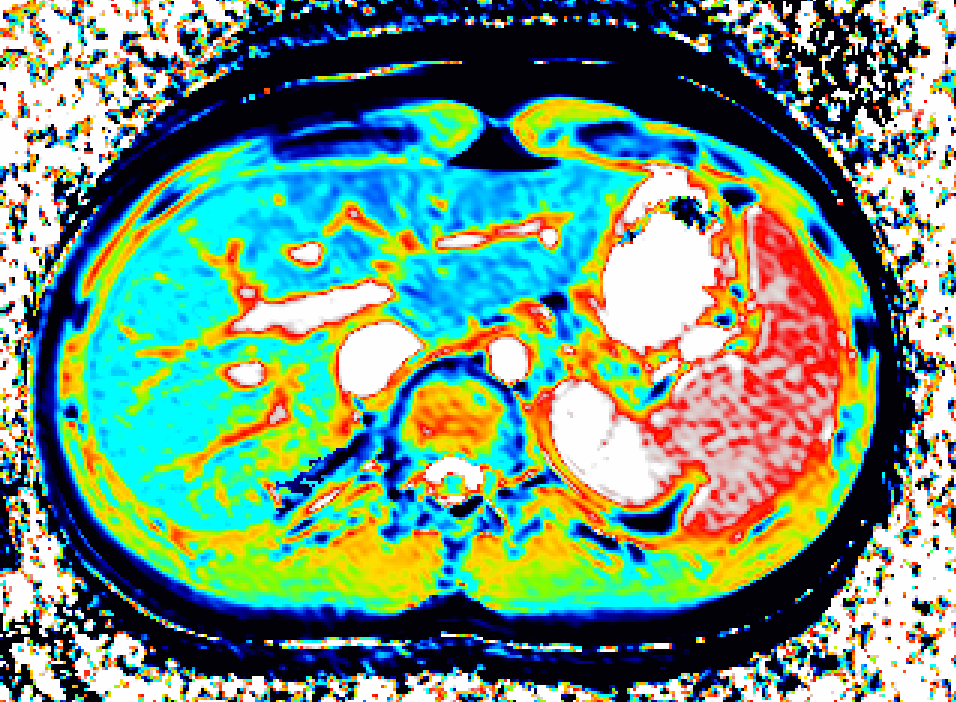
We use DCE-MRI of gadoxetate to measure liver transporter function and relaxation time measurements to assess tissue status. Imaging biomarkers provide specificity where uptake and efflux kinetics cannot be unambiguously determined from blood levels.
Drug uptake and efflux involve multiple transporters. If uptake is perturbed, drug clearance may change, leading to harmful overdosing or lack of efficacy. Drug-drug interactions and inhibited efflux can lead to drug-induced liver injury. Imaging biomarkers provide a direct window into these mechanisms.
The TRISTAN consortium developed and validated gadoxetate-based DCE-MRI biomarkers for DILI and DDI. Our specification has been accepted into the FDA's biomarker qualification program, supporting use in early phase clinical drug development.
Our platform of evidence includes in vitro, rat, and human studies of assay reproducibility and response to known transporter substrates, providing confidence in quantitative liver biomarkers for safety assessment.